For as long as she could remember, Dina Radenkovic was a meticulous planner, determined to leave as little as possible to chance. But Radenkovic — a physician, research scientist and biotechnology investor — felt gripped by uncertainty over how to plan for one of her most long-held goals: becoming a mother. She thought about freezing her eggs, but enduring two weeks of potentially uncomfortable and nauseating injections felt impossible: It would have meant missing work.
So in December 2022, Radenkovic, then 27, found herself alone in her kitchen in New York jabbing her abdomen with a needle, the first step in the arduous in vitro fertilization process. Egg retrieval alone requires roughly two weeks of daily shots that flood the body with hormones — often triggering a range of physical and emotional side effects — to induce the ovaries to produce as many mature egg follicles as possible.
But Radenkovic wasn’t trying to have a baby. She was experimenting on herself in a clinical trial that aimed to alter the experience of families struggling with infertility. If everything went according to plan, this would be Radenkovic’s only hormone shot.
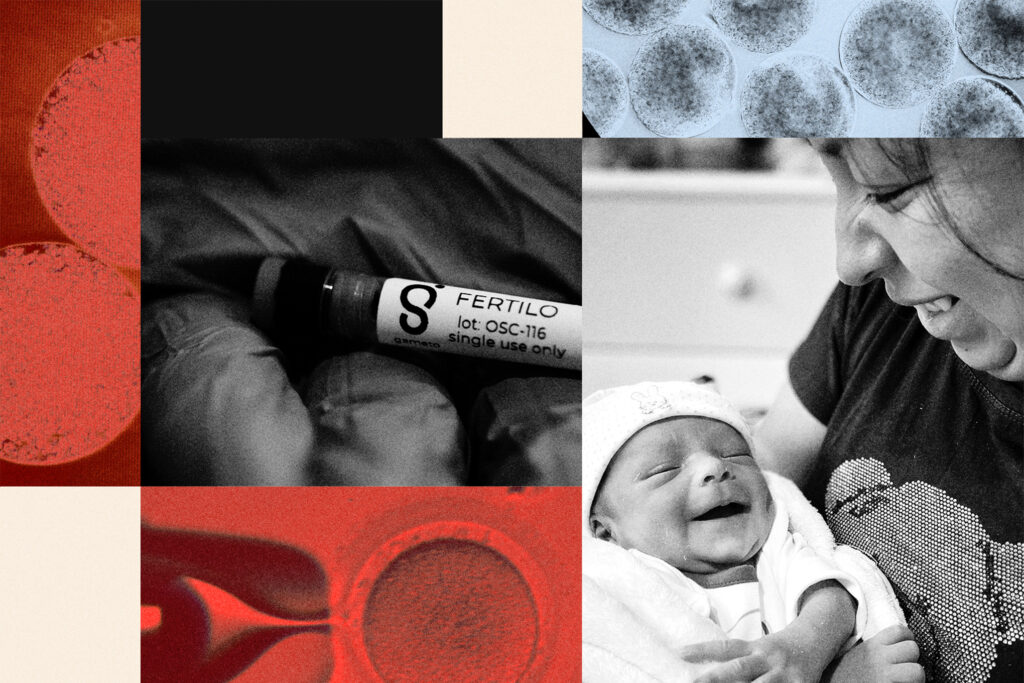
The injection was an early chapter in what has become Gameto, Radenkovic’s six-year-old company that is on the precipice of what some experts say may be one of the most significant innovations to IVF treatment in decades. Its first therapy, which was cleared for use in a dozen countries in the last year, bypasses expensive, often uncomfortable daily injections. Instead, doctors retrieve immature eggs using only one or two injections, then use living cells that mimic ovarian cells to mature them in a lab.
Gameto says its approach — replacing unhealthy ovarian cells with lab-made ones — could have implications far beyond fertility; it has the potential to ease a plethora of conditions associated with aging in women, including cardiovascular disease and osteoporosis.
The technique builds off a discovery that earned a Nobel Prize in 2012, showing how stem cells derived from human skin could be manufactured at scale and turned into any cell in the human body. Though the discovery, known as induced pluripotent stem cells, or iPSC, has myriad implications, including regenerating organs and treating thousands of diseases, actual clinical benefit hasn’t been realized.
No iPSC therapies are on the market in the United States, though dozens are in clinical trials for diseases such as diabetes, Parkinson’s and cancer.
In a little over a year, 11 babies have been born through Gameto’s first therapy, called Fertilo, a liquid soup of “ovarian support cells” that mimic the egg-growing function of cells in the ovaries. If Fertilo is approved by the U.S. Food and Drug Administration, it would be the first IVF treatment to realize the Nobel discovery domestically — and the first new IVF drug to be launched in the U.S. in over two decades.
“In our society, women have been told just to suck it up and deal with the pain,” said Radenkovic, now 30, in a videoconference from her home in Austin, where she had just given birth to her second child. (She never used the embryos that were created during the trial and ended up getting pregnant without IVF). She recounted being told by a fertility clinic director that he didn’t see much value in easing the burden of IVF.
“He said, ‘The women who come our clinic are so desperate — they would just jump off the roof if it meant getting pregnant,’” she recalled. “And I’m thinking, ‘But why would you make them do that?! Why would you make them jump off the roof?’”
Kara Goldman, a reproductive endocrinologist and director of fertility preservation at Northwestern University Feinberg School of Medicine, said that the barometer for success would be if Fertilo is able to produce pregnancies at the same rate as IVF. The company’s early trials have shown it to be comparable with IVF, and a Phase 3 clinical trial is underway in 15 U.S. clinics.
Goldman said she was “excited” about Gameto because it moved beyond “the current model of treating one disorder at a time … to target the underlying inefficiency of ovarian biology.”
The start-up is among a cohort of Silicon Valley ventures pushing the boundaries of reproductive biotechnology — energizing the long underfunded and under-researched arena of women’s health. In recent years, tech heavyweights including Facebook co-founder Dustin Moskovitz, OpenAI CEO Sam Altman, and the billionaire venture capitalist Peter Thiel have poured hundreds of millions into emerging “repro-tech.” Part of what Gameto said was its $127 million in funding came from Kristina Simmons, a reproductive health investor who said the company is “fundamentally about changing the timetable of the biological clock.”
While some projects are controversial and far from realization — “artificial wombs” that gestate babies outside the body; therapies that would enable two males to conceive — Gameto aims to address an immediate need at an opportune political moment. Rising infertility rates and concern about population decline are fostering a newly urgent conversation about barriers to parenthood, as are public figures such as Tesla CEO Elon Musk and Vice President JD Vance, who have encouraged women to have more children. Demand for IVF and other fertility services has exploded, pushing the Trump administration to announce policies that aim to broaden access and lower treatment costs.
If Gameto delivers on its early promise by making the most punishing part of IVF far less onerous, it could reshape how women approach fertility care. It also could make IVF possible for high-risk patients, including those with cancer, endometriosis or polycystic ovarian syndrome, for whom hormone treatments can be dangerous.
But Radenkovic aims to impact a broader medical challenge: ovarian aging. Countering it, she said, is key to helping women maintain health throughout life.
Scientists are just beginning to understand how declining ovarian function, which typically begins in a woman’s late 20s and accelerates in the years before menopause, might hasten diseases.
“The ovaries are far more than just egg factories; they are the canaries in the coal mine” for aging, said Jennifer Garrison, a neuroscientist and women’s health researcher at the University of California at San Francisco. The organ has been totally overlooked in the male-dominated longevity movement, she added.
Conditions such as cognitive impairment, loss of bone density, and cardiovascular disease manifest at a significantly higher rate in postmenopausal women, said Garrison, the former director of the Center for Healthy Aging in Women at the Buck Institute for Research on Aging. Ovarian aging is part of the reason women outlive men but spend more years in poor health than men do, she said.
Investors view the aging symptoms that accompany menopause as the next big opportunity in women’s health after fertility. The Trump administration recently galvanized that market by asking manufacturers to remove black-box warning labels for hormone replacement therapies. Gameto’s second therapy, which is called Ameno and is being experimented upon mice, is an under-the-skin implant that uses its youthful ovarian support cells to deliver tailored hormonal cocktails to patients — hopefully protecting menopausal woman from the accompanying symptoms of aging ovaries.
These stem-cell-derived treatments have the potential to open up a range of options for women throughout the second half of life, said Garrison, who advised Gameto early on but is not affiliated with the company.
The company says its fertility drugs are having a tangible impact.
While an IVF cycle is still an “emotional journey,” reducing the hormones required to retrieve eggs “dramatically reduces the physical side effects,” said Stacey Gouter, a 33-year-old event planner in Sydney who was 23 weeks pregnant in early December after using Fertilo.
Gouter, who struggled with infertility because of polycystic ovarian syndrome, said she “wouldn’t have even known she was doing IVF” during the egg-retrieval phase.
An overlooked organ
Vilma Durante, then an executive at Serono, a Swiss Italian company that is a leader in fertility treatments, had high hopes when the German pharmaceutical giant Merck KGaA bought the company in 2006. The $13 billion deal, which created Merck Serono, is the largest acquisition in the history of reproductive medicine. In the 1960s, Serono had pioneered the first hormonal fertility treatments, derived from the urine of postmenopausal women.
Now Merck was paying billions to own and market Serono’s highly profitable Gonal-F, a popular lab-produced IVF drug that went to market in the 1990s (Serono made other drugs, but fertility was its largest category at the time.) Durante hoped Merck’s resources would supercharge Serono’s research and development unit, which was working to find new fertility drugs and make Gonal-F less burdensome.
But five years after buying Serono, Merck shut down the R&D unit’s fertility projects, redirecting funding toward cancer and neurological disorders, Durante said. Merck spokeswoman Bettina Wassener said the company “continues to invest in innovation to advance fertility options and access for those looking to start or expand their family.”
The decision to cancel the unit was emblematic of the fits and starts that have characterized fertility research over the course of her career, said Durante, who today is vice president and head of the global fertility franchise for the Hungarian pharmaceutical company Gedeon Richter. Another large pharmaceutical manufacturer, Ferring, shut down its own fertility R&D unit two years ago.
Durante’s experience is borne out by data. Women’s health — a third of the category is reproductive health and the rest relates to other female-specific conditions — received 2 percent of the overall $41.2 billion in venture capital funding in health in 2023, far less than what is spent on diabetes, cancer and other health care challenges, according to the consulting firm Deloitte. Four percent of biopharmaceutical research and development is focused on female-specific conditions.
Many experts said there are business reasons companies don’t invest in research looking at women’s health. Gynecologic conditions receive low insurance reimbursement rates, and testing on pregnant women or reproductive tissue triggers liability concerns that can slow down trials and make them more costly.
Durante said that while Fertilo was “arguably the most significant innovation in the fertility drug space in the past two decades,” she still doubted that it would become broadly used, at least in the near term. Clinicians “tend to be quite conservative and are often reluctant to switch established protocols and drugs, particularly when a new approach is still relatively unfamiliar,” she said.
Radenkovic, who is Serbian, was only vaguely interested in these issues when she started studying medicine. Her father was an academic who researched the causes of miscarriage. Whenever a woman miscarried at the hospital in the midsize Serbian city where she grew up, the doctors would call her father to come pick up the specimen, and sometimes he would bring his daughters.
She would watch him diagram and dissect the dead fetal tissue, a scientific process that “captured my brain fully,” she said. It also gave her an awareness of her own ticking biological clock “since basically day one.”
In 2015, British TV personality Simon Cowell launched a technology talent-sourcing project modeled after his hit music talent show, “The X Factor,” in partnership with a network of Europe’s leading entrepreneurs and then-Google executive chairman Eric Schmidt. During her second year of medical school, at University College London, Radenkovic was invited, along with nine other aspiring, young tech and science founders, to pitch a start-up idea to a panel of business leaders.
There, she was introduced to Skype co-founder Niklas Zennström and Martín Varsavsky, an Argentine entrepreneur who would become pivotal to her work.
Varsavsky had recently launched Prelude, dubbed by Forbes magazine as “The $200 Million Startup That Wants To Stop The Biological Clock.” The company had been prompted by his wife’s struggles with IVF, though the couple went on to have three children. (He has seven children in total.) Varsavsky was acquainted firsthand with both the advances of IVF and the places where he viewed the industry was stuck. He told The Washington Post that it was clear to him early on that Radenkovic’s “entrepreneurial grit” would make her “unstoppable.”
Radenkovic and Varsavsky kept talking when she moved to New York to become a biotech investor, specializing in vetting the science behind companies working in longevity, a wellness and science trend seeking to extend lifespan and healthy years rather than focusing on any particular disease. One day, she was struck by a scientific paper about an intervention to improve the lifespans of mice. Even as the mice in the study were dying, she read, their ovarian cells remained young and plentiful.
Mice ovaries are not comparable with human ones because mice don’t menstruate. But as a math person, Radenkovic was fascinated by the huge mismatch between the rate of aging of the body overall and that of the ovaries. She started to think about how this might apply to human beings. When do the ovaries actually start aging, she wondered. Can this aging be measured as a rate or a formula? Does it occur follicle by follicle? Can it be treated?
She became “obsessed” with follicles, the tiny egg-containing sacs in ovaries, she said. While many successes in longevity are incremental and hard to measure, Radenkovic felt that extending the youthfulness of the ovaries would give tangible and meaningful benefits to people suffering from infertility and menopause.
She had vetted dozens of biotech companies, and she was astonished that such an important organ had been ignored. “No one was working on ovarian aging,” she said.
Through her scientific network, she learned that a laboratory at Harvard University, run by the prominent geneticist George Church, had been working on converting and reproducing stem cells into ovarian support cells, building on the process that won the 2012 Nobel Prize.
She partnered with Church’s lab, and a year later, Radenkovic and Varsavsky co-founded Gameto, along with one of the scientists from Harvard.
But even with the technique of converting the stem cell nailed down, she needed to manufacture cells at scale. Only one firm owned the intellectual property to do so, and Radenkovic negotiated to license it.
One of the first women to have a baby after taking Fertilo is Gaby Romani, a 37-year-old health administrator living in Lima, Peru. Like most IVF patients, she remembers the exact number of eggs she produced during her first retrieval, a robust 32. But IVF is a numbers and waiting game, and just four of those eggs matured. Only two survived to become embryos. She implanted one embryo, but the pregnancy didn’t take.
Romani had one embryo left. She was preparing to sell her car or take a loan out against her home to pay for another IVF cycle when her clinic told her about the Fertilo clinical trial. Though Romani had to take several hormonal injections to manage an underlying condition, it was far fewer than the earlier IVF round. The experience “was much easier and different from the first time, when I suffered so much,” she said.
One of the best things about having her eggs matured outside her body is that her work wasn’t disrupted by what felt like countless clinic visits. Her daughter was born this past June.
Radenkovic is moved by the lengths Fertilo patients have gone to become mothers, and she identifies with them. This is part of what drew her to Varsavsky, a highly successful entrepreneur “who had so many kids,” which made her feel like doing both was possible. Many of the investors and board members of Gameto are either IVF patients or IVF dads, said Simmons of Overwater Ventures, the lead investor. She felt so strongly about Gameto that she negotiated the company’s last funding round from the hospital room as she was about to give birth this summer.
As a non-U.S. citizen, Radenkovic can’t vote, though she is enamored enough with America that she flies a U.S. flag in front of her house and named one of her children Lincoln. She was impressed by the Trump administration for addressing both menopause treatments and IVF affordability by pushing drugmakers to slash the price of Gonal-F.
But she has mixed feelings about Vance and Musk advocating for women to populate the world with as many children as they can. If society really wants women to have more kids, then leaders should invest in science and family-friendly policies, such as parental leave, she said, rather than shaming women who don’t or can’t.
She remembers adrenaline keeping her up all night as she waited for the results of the first trial, praying the embryos created with her own Fertilo-grown eggs would be normal. Because the trial was known as double-blind, neither she nor Gameto knew what happened to her eggs after she donated them. If they were normal, she told herself, “I know women will want this. Because I am one of them.”
The post A company is trying to unlock a key to aging, in a long-overlooked body part appeared first on Washington Post.