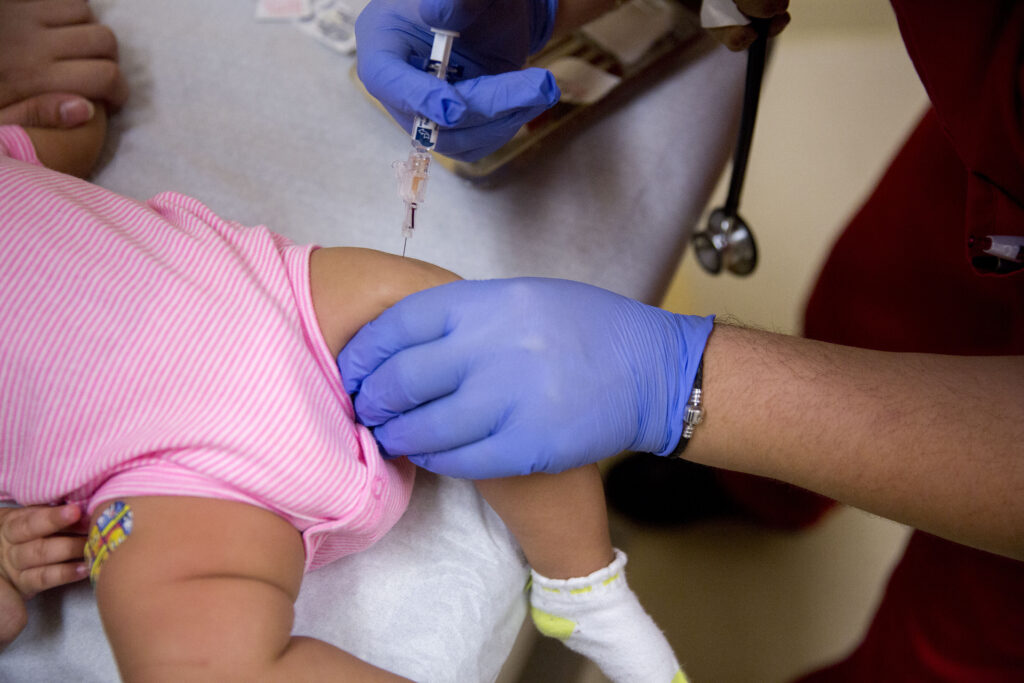
The Centers for Disease Control and Prevention approved a sweeping change to the nation’s childhood immunization schedule Tuesday, endorsing a recommendation from a federal advisory panel to drop the long-standing practice of giving all newborns a hepatitis B vaccine within 24 hours of birth.
The shift has intensified a national debate over infant vaccination and drawn swift resistance from pediatricians, state health departments and major medical groups.
The decision follows a contentious vote two weeks ago by the CDC’s Advisory Committee on Immunization Practices (ACIP), which recommended that parents of infants born to mothers who test negative for hepatitis B could, in consultation with their health care provider, delay the initial dose to at least 2 months of age.
“This recommendation reflects ACIP’s rigorous review of the available evidence,” acting CDC director Jim O’Neill said in a press release. “We are restoring the balance of informed consent to parents whose newborns face little risk of contracting hepatitis B.”
But the CDC did not adopt another recommendation by the committee that drew intense objections from pediatricians. The panel voted to recommend parents and health care providers consider blood tests to check levels of protective antibodies to decide whether a child needs additional doses of the three-dose hepatitis B vaccine. The agency is “reviewing” that recommendation, the press release said.
The committee’s votes prompted major outcry from medical associations, who say there is no evidence to delay the birth dose.
“Pediatricians are already reporting more parents declining to give their child this critical dose,” Susan Kressly, president of the American Academy of Pediatrics, said in a statement Tuesday. “As a pediatrician, this is heartbreaking when we have a vaccine that can prevent so many infections, and it is deeply disappointing to see the continued dismissal of expertise to inform recommendations that have broad implications on the health of America’s children.”
The AAP, joined by 66 medical organizations, said the committee’s recommendations are not based on science and “will harm children, their families and the medical professionals who care for them.”
Under the new guidance, the birth dose would still be recommended for infants whose mothers test positive for hepatitis B or whose status is unknown. Members of the committee argued that the universal recommendation was overbroad and unnecessary for mothers who test negative and that the risks of contracting the virus in early months of infancy were overstated. But pediatricians say the policy ignores the logistical and real-world challenges that led the United States to adopt universal newborn vaccination in 1991 — particularly the risk of missed or inaccurate maternal tests.
Several state health departments, including in Colorado, Connecticut, Maryland, Massachusetts, Michigan, New York and Rhode Island, regional health alliances and national medical organizations have explicitly reaffirmed recommendations for all newborns to receive a hepatitis B vaccine.
Major health insurance groups including the Blue Cross Blue Shield Association and AHIP have said they will continue to cover all vaccines that were recommended by ACIP at the start of 2025 through the end of 2026, including the hepatitis B birth dose.
The vaccine committee also proposed the use of antibody tests for parents to decide whether their children should receive all doses of the hepatitis B vaccine, prompting an uproar from pediatrician groups that said there was no data to justify that strategy. Clinicians also said drawing blood from babies is invasive and difficult.
“There is no evidence to delay that dose,” said Nola Jean Ernest, a pediatrician in rural southeastern Alabama. “I’ve never performed a hepatitis B antibody test on a healthy infant, and I would not know how to interpret the results.”
The revision to the hepatitis B vaccine recommendation comes amid broader changes in federal vaccine policy under Health Secretary Robert F. Kennedy Jr., a longtime critic of childhood immunization practices. Several pediatricians said they are now spending more time countering confusion and misinformation — including questions prompted by the vaccine advisers’ recommendation — without the institutional backing of federal health agencies they once relied on.
Ernest said five families with newborns have recently refused the birth dose for hepatitis B. After she explained the risks and benefits of the vaccine, two families got their infants the first dose in her office, she said.
Amid the confusion, here are answers to common questions about hepatitis B and the vaccine.
What is hepatitis B, and why are newborns at risk?
Hepatitis B is a contagious virus that attacks the liver and can cause complications later in life, including chronic liver disease, cirrhosis and liver cancer. Up to 90 percent of infants infected at birth will develop lifelong infection. The birth dose of the vaccine provides critical protection if a mother’s infection status is unknown, testing was inaccurate or she becomes infected after she was tested. Childbirth is a major risk factor for transmission because the virus is present in vaginal fluid and blood.
Hepatitis B can also spread through tiny amount of virus on toothbrushes or nail clippers, or contact with caregivers or household members. Screening practices are imperfect in the United States, and many people with chronic infections do not know it.
What is the hepatitis B vaccine, and why is it given in three doses?
The vaccine trains the immune system to recognize and fight the virus. Infants need three doses because the final shot acts as a booster that solidifies long-term immunity, providing 95 percent protection. “Completing the hepatitis B vaccine series gives the infant the best chance for protection,” said Noele Nelson, a physician and hepatitis expert at Cornell University who was a senior author on the CDC’s previous guidelines for the vaccine.
Critics say the panel did not present compelling data showing that a single early dose provides enough protection to safely postpone the rest of the series.
Is there evidence that delaying the birth dose is safe?
Infants infected because of missed or inaccurate tests were a key reason the universal birth dose was adopted.
The universal hepatitis B birth dose was adopted after risk-based strategies proved insufficient and thousands of babies were still infected yearly. Following its implementation, hepatitis B transmission at birth declined sharply, with only a small number of cases now reported each year.
The post CDC approves major child vaccine change, rejects controversial one appeared first on Washington Post.